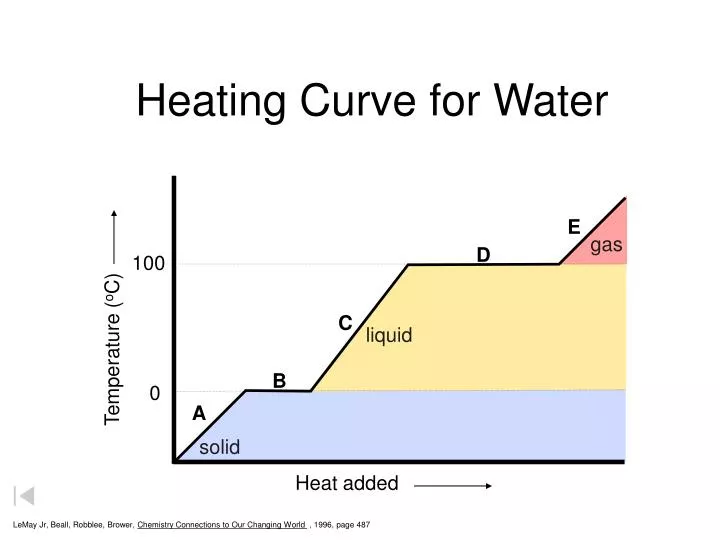
Temperature Vs Heat Added Graph . the heat needed to change the temperature of a given substance (with no change in phase) is: The system is constructed so that no vapor forms while ice warms to. Q = m × c × δt (see previous. temperature is a measure of average kinetic energy per molecule in a substance while heat is the form of energy. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heat graphs (or temperature vs. the heating curve for water shows how the temperature of a given quantity of water changes as heat is added at a constant. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. how to draw and read temperature vs. heating and cooling curves are graphs. figure 11.10 a graph of temperature versus added energy.
from www.slideserve.com
temperature is a measure of average kinetic energy per molecule in a substance while heat is the form of energy. how to draw and read temperature vs. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Q = m × c × δt (see previous. the heating curve for water shows how the temperature of a given quantity of water changes as heat is added at a constant. heating and cooling curves are graphs. The system is constructed so that no vapor forms while ice warms to. figure 11.10 a graph of temperature versus added energy. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the heat needed to change the temperature of a given substance (with no change in phase) is:
PPT Heating Curve for Water PowerPoint Presentation, free download
Temperature Vs Heat Added Graph The system is constructed so that no vapor forms while ice warms to. temperature is a measure of average kinetic energy per molecule in a substance while heat is the form of energy. the heating curve for water shows how the temperature of a given quantity of water changes as heat is added at a constant. heating and cooling curves are graphs. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Q = m × c × δt (see previous. Heat graphs (or temperature vs. figure 11.10 a graph of temperature versus added energy. how to draw and read temperature vs. The system is constructed so that no vapor forms while ice warms to. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the heat needed to change the temperature of a given substance (with no change in phase) is:
From sciencing.com
Heat vs Temperature What are the Similarities & Differences? (w/ Graph Temperature Vs Heat Added Graph heating and cooling curves are graphs. how to draw and read temperature vs. Heat graphs (or temperature vs. the heating curve for water shows how the temperature of a given quantity of water changes as heat is added at a constant. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a. Temperature Vs Heat Added Graph.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Temperature Vs Heat Added Graph figure 11.10 a graph of temperature versus added energy. Heat graphs (or temperature vs. Q = m × c × δt (see previous. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for. Temperature Vs Heat Added Graph.
From heat-transfer-thermodynamics.blogspot.com
Heat Transfer and Applied Thermodynamics Specific Heat Ratio Temperature Vs Heat Added Graph figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the heat needed to change the temperature of a given substance (with no change in phase) is: figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water.. Temperature Vs Heat Added Graph.
From www.worldwisetutoring.com
Heating and Cooling Curves Temperature Vs Heat Added Graph Heat graphs (or temperature vs. how to draw and read temperature vs. Q = m × c × δt (see previous. the heat needed to change the temperature of a given substance (with no change in phase) is: figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of. Temperature Vs Heat Added Graph.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Temperature Vs Heat Added Graph Q = m × c × δt (see previous. the heat needed to change the temperature of a given substance (with no change in phase) is: The system is constructed so that no vapor forms while ice warms to. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of. Temperature Vs Heat Added Graph.
From allisonrswankxo.blob.core.windows.net
Heating Cooling Curve Of Water Temperature Vs Heat Added Graph temperature is a measure of average kinetic energy per molecule in a substance while heat is the form of energy. heating and cooling curves are graphs. how to draw and read temperature vs. Heat graphs (or temperature vs. the heating curve for water shows how the temperature of a given quantity of water changes as heat. Temperature Vs Heat Added Graph.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Temperature Vs Heat Added Graph how to draw and read temperature vs. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. temperature is a measure of average kinetic energy per molecule in a substance while heat is the form of energy. the heat needed to change the temperature of a. Temperature Vs Heat Added Graph.
From printablezonemarrow.z13.web.core.windows.net
Heating And Cooling Curves Explained Temperature Vs Heat Added Graph figure 11.10 a graph of temperature versus added energy. Q = m × c × δt (see previous. how to draw and read temperature vs. heating and cooling curves are graphs. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The system is constructed so. Temperature Vs Heat Added Graph.
From www.met.reading.ac.uk
PPLATO FLAP PHYS 7.4 Specific heat, latent heat and entropy Temperature Vs Heat Added Graph how to draw and read temperature vs. Heat graphs (or temperature vs. Q = m × c × δt (see previous. figure 11.10 a graph of temperature versus added energy. heating and cooling curves are graphs. The system is constructed so that no vapor forms while ice warms to. the heating curve for water shows how. Temperature Vs Heat Added Graph.
From chart-studio.plotly.com
Heat vs. Change in Temperature Direct Relationship scatter chart Temperature Vs Heat Added Graph the heat needed to change the temperature of a given substance (with no change in phase) is: The system is constructed so that no vapor forms while ice warms to. how to draw and read temperature vs. figure 11.10 a graph of temperature versus added energy. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature. Temperature Vs Heat Added Graph.
From www.teachoo.com
For any substance, why does the temperature remain constant during the Temperature Vs Heat Added Graph figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. figure 11.10 a graph of temperature versus added energy. heating and cooling curves are graphs. Web. Temperature Vs Heat Added Graph.
From www.texasgateway.org
14.3 Phase Change and Latent Heat Texas Gateway Temperature Vs Heat Added Graph the heating curve for water shows how the temperature of a given quantity of water changes as heat is added at a constant. temperature is a measure of average kinetic energy per molecule in a substance while heat is the form of energy. The system is constructed so that no vapor forms while ice warms to. figure. Temperature Vs Heat Added Graph.
From www.youtube.com
Learn the Time/Temperature Graph for Water YouTube Temperature Vs Heat Added Graph Heat graphs (or temperature vs. how to draw and read temperature vs. Q = m × c × δt (see previous. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. figure 11.10 a graph of temperature versus added energy. the heating curve for water shows. Temperature Vs Heat Added Graph.
From chemnotcheem.com
2019 O Level Pure Chemistry Practical Paper Chem Not Cheem Temperature Vs Heat Added Graph figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heat graphs (or temperature vs. Q = m × c × δt (see previous. how to draw and read temperature vs. temperature is a measure of average kinetic energy per molecule in a substance while heat is. Temperature Vs Heat Added Graph.
From chemistry101efhs.weebly.com
Heat vs. Temperature Chemistry 101 Temperature Vs Heat Added Graph figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the heat needed to change the temperature of a given substance (with no change in phase) is: figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water.. Temperature Vs Heat Added Graph.
From askfilo.com
The graph below shows the heating curve for a pure substance. The tempera.. Temperature Vs Heat Added Graph heating and cooling curves are graphs. the heat needed to change the temperature of a given substance (with no change in phase) is: figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heat graphs (or temperature vs. temperature is a measure of average kinetic energy. Temperature Vs Heat Added Graph.
From shaunmwilliams.com
Chapter 10 Presentation Temperature Vs Heat Added Graph figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. temperature is a measure of average kinetic energy per molecule in a substance while heat is the form of energy. the heat needed to change the temperature of a given substance (with no change in phase) is:. Temperature Vs Heat Added Graph.
From chem.libretexts.org
5.5 Heating Curves and Phase Changes Chemistry LibreTexts Temperature Vs Heat Added Graph how to draw and read temperature vs. heating and cooling curves are graphs. figure 11.10 a graph of temperature versus added energy. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the heating curve for water shows how the temperature of a given quantity. Temperature Vs Heat Added Graph.